Goals for this unit:
1. Understand essential theoretical concepts of movement of a charged particle in an electric field.
2. Know types of media commonly used for electrophoresis and the difference between zonal and boundary methods
3. Be familiar with common applications:
4. Other Practical Aspects (tracking dyes / staining )
(Some of the electrophoresis notes given below are modified in part from notes by Terry Frey - San Diego State Univ.)
Ff = f v ( f = frictional coefficient); when Ff = -F, no more acceleration
==> molecule has reached its limiting velocity.
4. Define a mobility per unit field, U
5. Stokes Law: For a spherical molecule of radius, r, and charge z e (e = elementary charge, charge on 1 electron)

where rh is the radius of a sphere of equal volume, and h is viscosity (~0.01g/cm-sec).
Electric field actually felt by the macromolecule is difficult to evaluate due to the fact that the macromolecule is a very large ion in solution with many small counterions. Extreme situations:
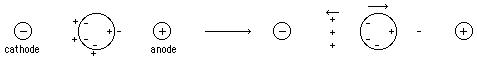
- Tube Gels -- polymerize in glass tubing ==> cylindrical shape
- Slab Gels -- polymerize between glass plates
- sample is applied in a zone (small region: a spot on moistened paper or a band on a gel) and an applied field causes the molecules to separate into zones based upon different U's.
- need some way of stabilizing the zones to prevent mechanical mixing (from vibrations) or convection mixing (from temperature differences -- a particularly severe problem with resistance heating caused by the electric field).
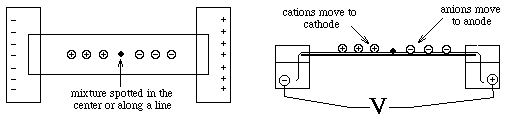
For example - Paper Electrophoresis
- a strip of paper is kept moist with buffer to make it electrically conductive; ends are dipped into buffer solutions containing electrodes across which an electric potential is applied
- Used primarily for separation of small molecules ==> must use a high voltage, otherwise they diffuse too rapidly ==> paper must be cooled (usually by water)
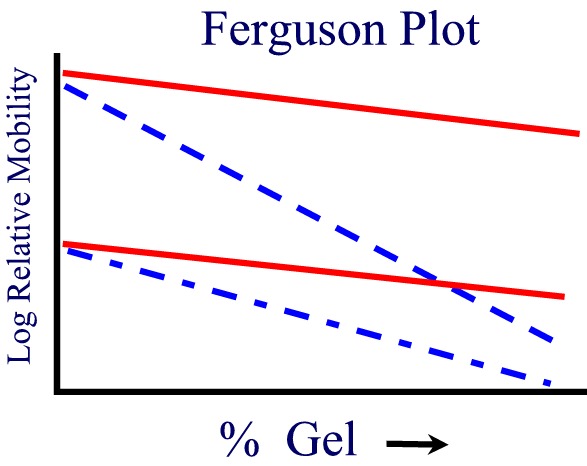
Log relative mobility vs. % Gel conc. log U = log Uo - K[C]
Consider simple (conformationally) molecules moving free in solution in the presence of an electric field.
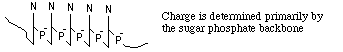
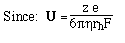 --> both numerator and denominator contain terms
directly proportional to M
--> both numerator and denominator contain terms
directly proportional to M ==> M terms cancel out and the electrophoretic mobility
==> U, should be ~ independent of the
size of the nucleic acid.
(N. Davidson at CalTech measured U for nucleic acids without a supporting gel, and found that it is constant from 1 nucleotide up to 1.7 x 105 nucleotides!)
2. How can we use electrophoresis to separate molecules?
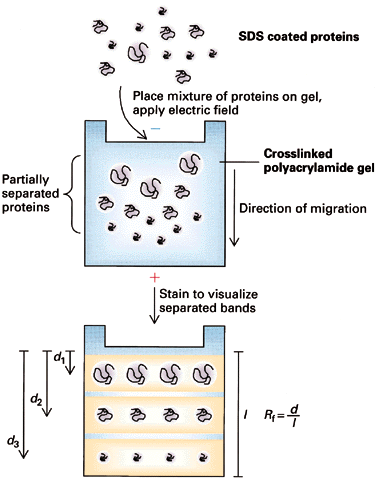
==> Do Electrophoresis in a gel matrix -- the gel sieves the molecules
large molecules move slowly because they have difficulty going through the pore
small molecules move rapidly because their freedom is not restricted
3. Choice of GelU is measured as the "relative" distance traveled, Rf = R / Rmax
- How is pore size related to gel concentration? We can adjust the gel concentration to produce a pore size appropriate for the sizes of molecules we wish to separate.
- 7.5% (45K-400K) / 10% (22K-300K) / 12% (13K-200K) / 15% (2.5K-100K)
Experimentally, we find that Rf depends upon log M==> Rf = b - a log M where "a" and "b" are constants determined (primarily) by the gel; they're measured empirically by plotting Rf for molecules of known M and making a calibration curve. Note: the relationship is linear only over a limited range of log M.
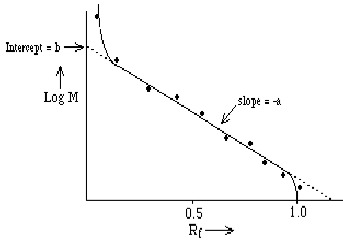
- Agarose: 0.2 % for Nucleic Acids up to M = 150 x 106
0.8 % for Nucleic Acids up to M = 50 x 106- Polyacrylamide: for smaller Nucleic Acids; choose % acrylamide to produce correct size pore
4. Common Applications - sequencing gels (Fig 1, Fig 2) / Southern blots (see animation 2)
- amino acids composition
- pH;
- net charge can be + or - or 0
What is needed is a way of modifying proteins so z is proportional to M (as is the case with nucleic acids).
3. SDS PolyAcrylamide Gel Electrophoresis -- SDS PAGE
- Sodium Dodecyl Sulfate = Sodium Lauryl Sulfate: CH3(CH2)11SO3- Na+
A detergent that contains a hydrophobic tail region [ CH3(CH2)11 ], attached to a hydrophilic group, SO3- Na+, making it amphipathic. It is a very strong detergent which denatures proteins by binding to the polypeptide backbone.- Measurements show that most proteins bind 1.4 gm SDS/gm protein with very little variation (except for some membrane proteins; membrane proteins are very hydrophobic and may bind more SDS)
==> The charge on an SDS-protein complex is determined almost entirely by SDS --> -1 charge for each SDS. Since the amount of SDS bound is determined by the size of the protein and all protein/SDS micelles are anionic ==> z is directly proportional to M
Hydrodynamic studies show that the shape of an SDS/protein complex is a rod or prolate ellipsoid of ~18Å diameter and a length proportional to M
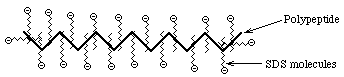
Electrophoresis of SDS/protein micelles through a polyacrylamide gel should separate them according to M (~ nucleic acids) ==> Rf = b - a log M
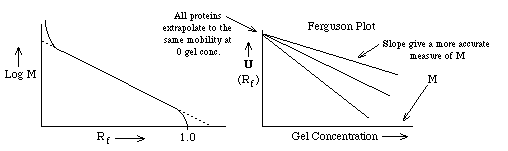
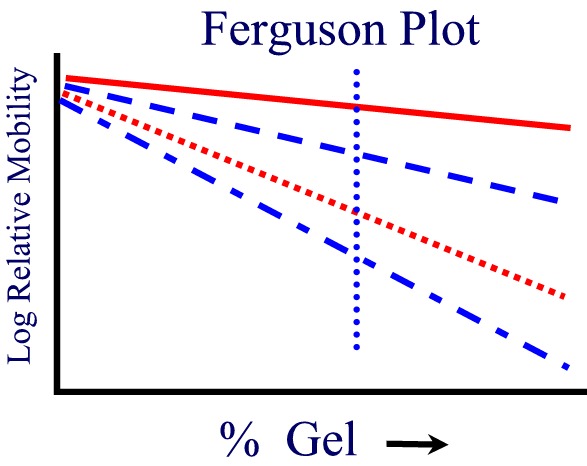
If one plots the mobility for different proteins as a function of gel concentration, a Ferguson Plot, one finds that the mobilities of all proteins extrapolate to the same value at [gel conc.] = 0 as expected. The slope of the mobility of a single protein provides another more accurate method of estimating its molecular weight from SDS PAGE.
They experience different potentials: VU >> VL
Ohms Law: V = R i: i = current and is the same for both layers, R is the resistance. RU >> RL, because:
where ci = concentration; mi = mobility; zi= charge on the ith ion. ==> RU >> RL because mU is smaller than mLThus, if cU and cL are chosen appropriately, VU will be enough larger than VL to compensate for lower mobility of ions in the upper zone
==> 2 zones move at the same speed with a sharp boundry between
them.
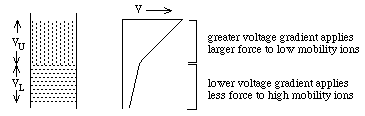 .
.
Why a sharp boundry? (1) if a mobile lower zone ion drifts into the upper zone, it immediately experiences a higher potential, VU, and speeds up until it reaches the lower zone where the lower potential, VL, causes it to slow down again. (2) if a low mobility upper zone ion drifts into the lower zone, it immediately experiences a lower potential, VL, which causes it to slow down until it drifts back into the upper zone.
What about proteins? Choose upper and lower zone ions so that: mU < mproteins /SDS < mL
Cl- vs. proteins / SDS vs. Gly [mobility of Gly varies with pH; (pH 6.7, Gly ~0 / > pH 9.0, Gly ~ -1)]
Upper "stacking gel" - proteins / SDS > Gly ~(0)
Lower "running gel" - Gly ~(-) > proteins / SDS
==> proteins will be concentrated at the interface into thin zones stacked in order of protein mobility. Note: all this assumes electrophoresis in a "stacking" gel with large pores which do not inhibit protein movement.
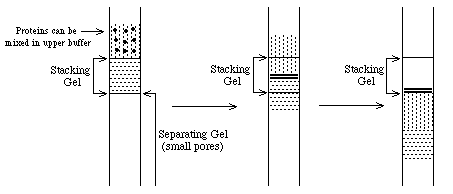
When the thin bands of proteins reach the "separating" gel, their mobility decreases dramatically and the upper buffer ions pass them. At this point, the proteins are separated according to their individual mobilities (sizes). The whole point of this buffer system is to concentrate the proteins into very thin zones before separating them.
Common SDS page protocol / web info on SDS PAGE technique
All protein carry charges that vary from a net positive charge at low pH (-COOH and -NH3+ forms of acidic and basic functional groups), through 0 at some intermediate pH, to a net negative charge (-COO- and -NH2 forms) at high pH.
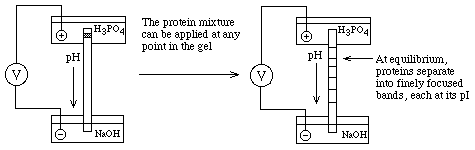
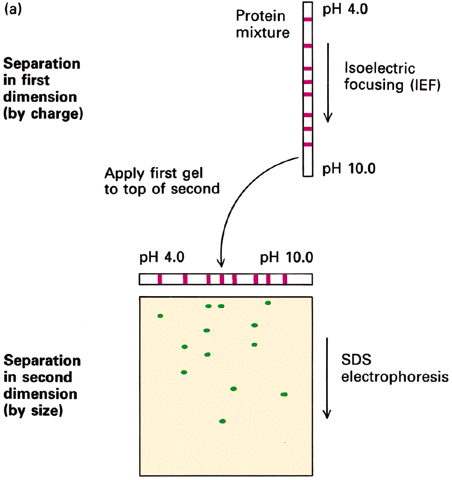
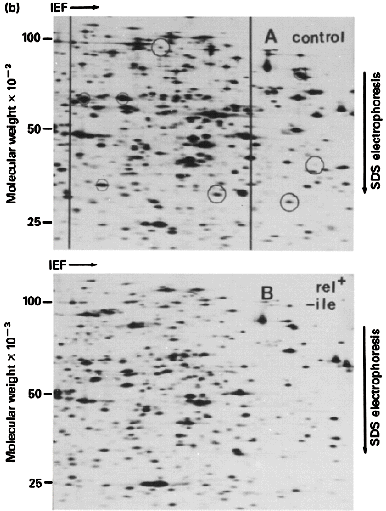
- SDS-PAGE -- non-reducing conditions (-S--S- bonds intact) + reducing conditions
Tracking Dye [ bromophenol blue (+) / methylgreen (-) ]
Staining [ Coomassie Blue / Silver / SYPRO orange / Ethidium bromide]