Analytical ultracentrifugation is a means for determining
molecular weight and the hydrodynamic and thermodynamic properties of
a protein or macromolecule. Sample purity, molecular weight
determination in the native state, analysis of associating systems,
determination of sedimentation and diffusion coefficients and ligand
binding can all be determined using this versatile instrument.
The instrument itself (Beckman Optima XL-A) spins a rotor at a
controlled speed and temperature under vacuum while recording the
concentration distribution at set times. The rotor may spin at speeds
up to 60,000 rpm (ª250,000 x g). Special cells that can
withstand the high gravitational field and allows the passage of
light through the sample are used. The sample itself is contained
within a sector-shaped cavity sandwiched between two windows of
quartz or sapphire. This cavity is contained within a centerpiece of
aluminum alloy, reinforced epoxy, or a polymer (Kel-F). Double sector
cells are also used so that the absorbing components of the sample
solvent can be taken into account. These cells also allow the
measurement of sedimentation coefficient differences and of diffusion
coefficients. These centerpieces can have a pathlength of 3 to 12 mm
which combined with selectable wavelengths, allows the examination of
a wide range of sample concentrations.
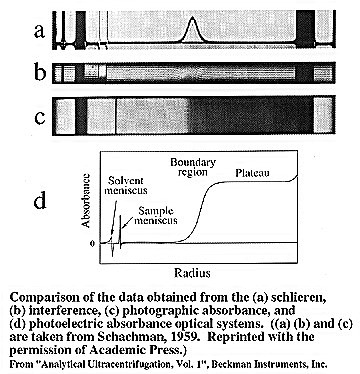
The data obtained from the instrument is a record of the
concentration distribution. This is accomplished by obtaining a set
of concentration measurements at different radial positions and at a
given time. This is achieved using either refractometric methods or
photoelectric absorption measurements.
Refractometric
Early instruments used refractometric methods to obtain concentration
distributions, using either the Schlieren optical system or Rayleigh
interference optics. These methods have the disadvantage that they
measure concentration difference relative to a reference point.
However, they are still used in some cases due to their applicability
to samples with little optical absorbance.

Absorbance
The measurement of absorbance allows increased sensitivity and high
reproducibility. With the absorption optics, the absolute
concentration is available at any point rather than a concentration
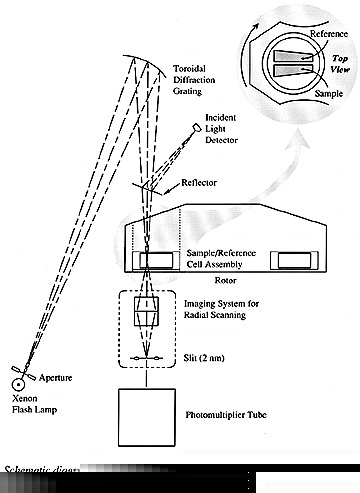
difference with respect to a reference point. The absorbance optical
system for our instrument is shown below. The xenon flash lamp allows
the use of wavelengths from 190 nm to 800 nm. This lamp is fired as
the selected sector passes the detector and the measured light is
normalized against a reflected small fraction of the incident light.
A slit below the sample can move to allow the sampling of different
radial positions. To help reduce noise, multiple readings are usually
taken from a single position and averaged.
In addition to the collection of concentration distribution,
several other quantities may be required. The density of the solvent
and the partial specific volume of the solute are required for
molecular weight determination. The viscosity of the solvent and its
temperature dependence is required in order to account for the
effects of solvent and temperature on sedimentation behavior.
With analytical centrifugation, the two basic types of experiments
are sedimentation velocity and sedimentation equilibrium.
Sedimentation Velocity
The solute particles pellet at the bottom of the cell, producing a
depletion of solute near the meniscus and the formation of a boundary
between the depleted region and the uniform concentration of the
sedimenting solute. This determines the rate of movement of a solute
under a centrifugal field. The rate of movement is equal to the
sedimentation coefficient (s), which depends directly on the mass of
the particle and inversely on the frictional coefficient. This gives
a measure of effective size of the particle.
Allows determination of:
Sedimentation Equilibrium
The solute particles do not pellet at the bottom of the cell. Instead
they redistribute over time with increasing concentration as the
distance from the center of rotation increases. After an appropriate
period of time, the process of diffusion equals the process of
sedimentation (called the sedimentation equilibrium). Measurement of
the solute concentration at different time points leads to the
determination of the molar weight of the sedimenting solute.
Allows calculation of:
References:
Beckman, Model XL-A Training Guide, Beckman Instruments, Inc., Palo
Also, CA, 1993.
Ralston, G. Analytical Ultracentrifugation Volume 1. Beckman
Instruments, Inc., Fullerton, CA, 1993.
Analytical Ultracentrifugation Submission Form (PDF)